Longevity at Vitalia. What is happening & who is causing it?
Debriefing the January LongBio conference
LongBio. A catchy term that stands for both ‘Longevity Biotech’ and ‘Long-term Bio-accelerationism & Human Enhancement.’
The LongBio conference (part 1) was a 3-day long longevity-centered event on the island of Roatan, in the city of Vitalia.
What’s Vitalia you ask? Learn more about the city of life in this dedicated article!
If you want to learn more about the experience of co-organizing the conference together with Sebastian Brunemeier and Ryan Spangler, there is an article on that too. ;)
The event brought together 50+ speakers, each with unique affiliations and expertise. This should come as no surprise, given the breadth of the longevity field: from stem cell transplantations, intelligence enhancement, regulatory breaches, and biohacking, these people can talk in depth about anything.
In this article, I will highlight (some of) the speakers of Day 1 and Day 3, to the extent to which they pointed out the main ideas the industry is built upon and the problems some companies are tackling in the space.
Some of the affiliations of the LongBio Conference Speakers, Jan. 2024.
DAY 1. Intro to longevity
Andrea Maier, on Healthy Longevity Medicine Clinics
As CEO of Chi Longevity, Andrea uncovered the limitations of such clinics: they are poorly regulated because there are no established standards (for training, certification, etc.), while in the infancy of the field. To find out what the clinics actually look for and do, a survey was conducted. Here are some of the results:
Q: When does a clinic define itself as a Longevity clinic?
A: Most often, when it helps manage human biological age.
Q: What hallmarks of aging do the clinics tackle?
A: Commonly, inflammation and epigenetic changes
Q: What treatments do the clinics offer?
A: Hormone replacements and lots of esthetics
Q: Common limitation?
A: The lack of associated technology: many don't have mobile apps or other tech to keep the patients in touch with the clinics and the data.
Together with Brian Kennedy, Andrea leads the Healthy Longevity Center at NUS (Singapore) aiming to identify diagnostics and interventions longevity clinics can and should provide to the public, for proper longevity healthcare.
I would’ve loved to hear how many longevity clinicians are out there and where.
Emily Welsch, on strategies to make clinical trials faster and more cost-effective
Clinical trials fail a lot due to lack of patient enrollment; they take enormous amounts of time, become more and more challenging and demanding, and compete over the few investigators the field has
Strategies: optimize trial design by adjustments mid-trial, based on interim results; keeping protocols simple; allow for remote/hybrid participation; and many others.
(Maybe AI could help with the implementation of all these strategies?)
Yuta Lee, updates on cell & gene therapy
By definition,
Cell therapy = transplant human cells to repair damaged tissue / cells
Gene therapy = insert genes to replace defective alleles
CAR-T = harnessing the power of (targeted) immune cells to treat cancer; high remission rates (77%)!
In practical terms,
The FDA keeps approving more and more cell and gene therapies per year, with an estimated 17 new therapies to be approved in 2024.
The cost of production, although considerable, is by far smaller than the lifetime cost of associated standard care.
For those who don’t know the term transhumanism, it’s about time to look it up. Gene and cell therapies are one strong tool to achieve human enhancement goals.
Aubrey de Grey, on how to frame the story around aging
People don’t like getting sick; over the centuries, we developed various behavioral ways to avoid disease and death, especially premature death. What’s the difference in treating aging? Why are the late-life problems not tackled enough?
When it comes to aging, most people think that many things go wrong in life, more or less at the same time, which leads to a complexity impossible to address.
Similarly, if you ask people to categorize the ways in which they can become sick, they think of infectious, chronic, and congenital diseases. Aging, on the other hand, is incorrectly considered separate from diseases, untreatable and obscure.
I absolutely love when people talk about the power of storytelling and reframing. The right choice of words leads to the right perceptions and attitudes.
Jonathan Anomaly, on predicting lifespan for embryos
Longevity is polymorphic, i.e., it is a trait influenced by a plethora of genes
Individual disease predictors are trained on cohorts of genomic data, in combination with their disease status
Using these predictors on the embryo data, you can obtain one index, the “Polygenic Longevity Index”, that estimates the longevity of the embryo, if implanted
Should predicted longevity become a mainstream factor in the choice of embryo implantation?
Alejandro Ocampo, on the basics of partial reprogramming
Partial (epigenetic) reprogramming refers to the restoration of cells to a more youthful state, by epigenetic markers.
A central question behind epigenetic reprogramming is whether epigenetic dysregulation is a driver of aging (correlations between the two are insufficient)
The field evolved since 2016, with many new companies, like Altos, Life, and Retro continuously emerging
The translational barriers are nonetheless strong:
safety: Yamanaka factors can be oncogenes
efficacy: need to deliver these factors externally
cost: high cost of development
regulatory: low approval rates
logistics: complex distribution of the Yamanaka factors.
With such backing and strong companies, partial reprogramming remains a huge hope for the longevity space.
Alex Tabarrok, on the efficacy of the FDA
Alex’s talk was a brilliant storytelling experience. He brought to our attention the sensor pad – a device designed to help women detect breast cancer early stage – classified as “high risk” by FDA because… no other device like it existed on the market.
Countries outside the US adopted it fast. Nonetheless, the FDA asked for clinical trials which would have taken extraordinary amounts of time, resources, and would have basically waited for women in these clinical trials to develop breast cancer in order to prove the (in)efficiency of the device. The company producing the sensor pads started giving away the device, and only years afterwards the device was approved due to positive and encouraging reviews from these users.
Key takeaways:
“drug lag and drug loss lead to many deaths” (i.e., drugs that are not available on the market to patients because they are not approved in time etc. by the FDA)
We need to get rid of regulatory nationalism: if a drug is approved in one country, it should be at least considered in others
new uses for old drugs are discovered all the time and off label use is very common; medical knowledge advances faster than the FDA
patients are heterogeneous, which implies doctors need to have options to prescribe
off label prescribing is like prescribing a new drug that only goes through FDA-required safety check, without the efficacy check
Image generated with the help of DALL E.
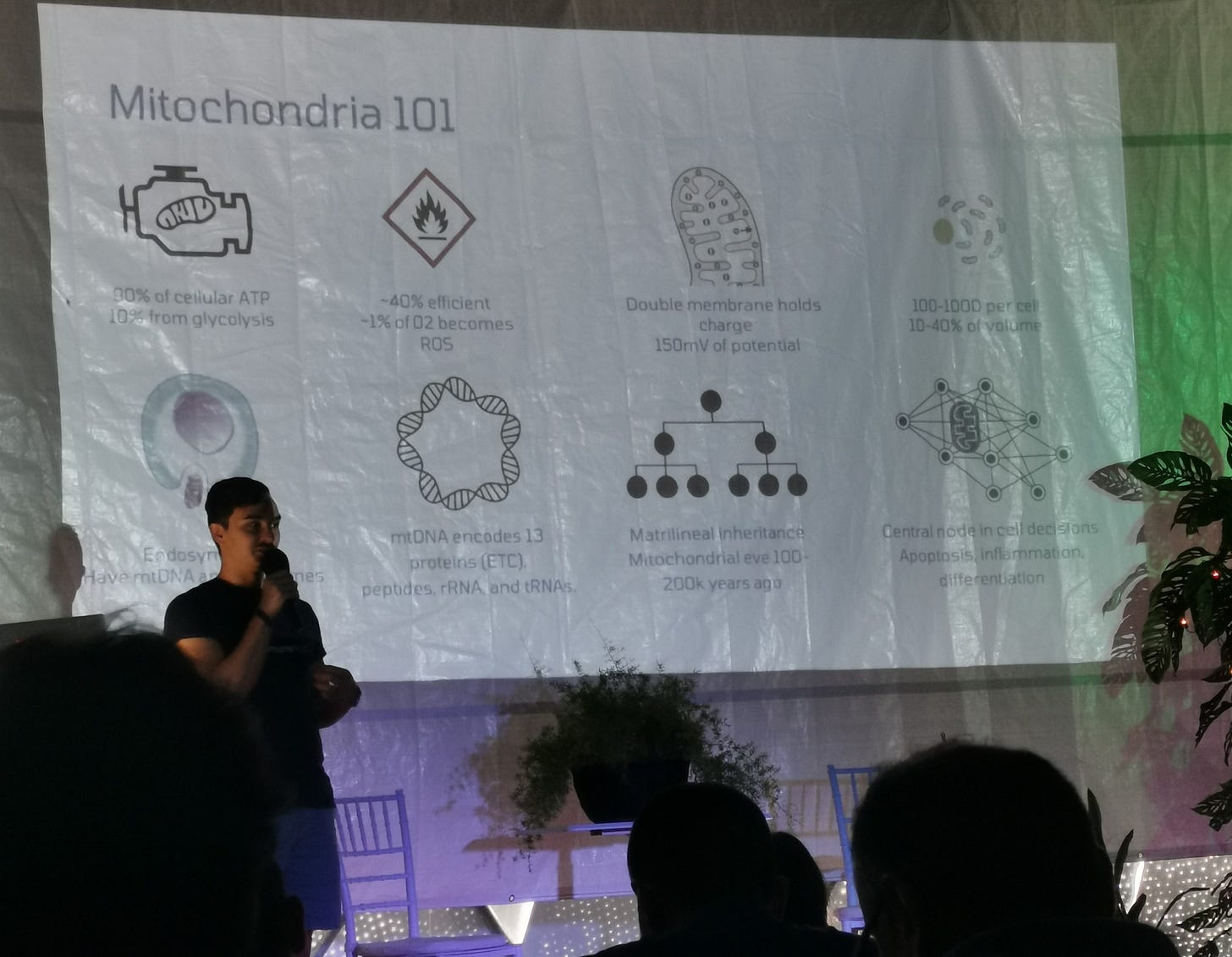
Luis Rios, on mitochondria in aging
There are primary drivers of aging in mitochondria, like mtDNA damage accumulation, decreased mitochondrial biogenesis and mitophagy
In turn, these lead to reduced ATP (and thus cellular energy),fatty acid oxidation, increased ROS production, chronic inflammation, and many other cellular dysfunctions
We are attempting to treat these negative consequences via small molecules that either increase mitophagy and biogenesis, decrease oxidative stress, or minimize mtDNA damage
Other ways to mediate these effects are by administering anti-inflammatories (e.g. senolytics), cell/tissue replacement therapies, fasting mimetic targets to increase turnover (AMPK, mTOR, HIF-1a, Sirtuins, PGC-1a, etc.), epigenetic reprogramming, etc.
Matt Scholz, on his biohacking journey
From tasing himself in the muscles to side effects of biohacking, Matt’s entertaining journey was too engaging for me to take notes on. Simply too good.
Josh Mitteldorf, on methylation aging clocks
There are two sides of aging biological research:
Aging is considered the result of self-inflicted damage and programmed shut-down of the body’s repair mechanisms. Thus, “younger” means “less self-destruction”, or, “younger” = “better”.
Aging is deemed a passive accumulation of damage in the body that gradually overwhelms the body’s ability to repair. Thus, “younger” = “less repair mechanisms active”, or, “younger” = “worse”, in terms of methylation patterns.
Methylation aging clocks should measure a combination of the two, i.e., as little self-destruction and as much repair capacity.
Since growth, development, puberty are on a timetable, so could be aging. Exosomes could be at play in this mechanism, as means of communication for age-related information
DAY 2. Big Ideas
As the title says, this was the time for the showcase of Big Ideas in the space. Some key ideas featured were
Vitalism (by Nathan Cheng),
the Blueprint longevity protocol (by Bryan Johnson),
the future of designer babies (by Max Berry),
Próspera as a new governmental form (by Próspera CEO Erick Brimen), and
tackling DNA mutations to increase healthy lifespan (by Chris Bradley @Matter Bio).
DAY 3. Company Showcases
Some highlights:
Kelsey Moody, Ichor Life Sciences
Purpose: Adhere to the SENS damage repair hypothesis, with portofolio companies targeting each of the SENS programs
Examples: LysoClear: company tackling enzyme therapy for age-related macular degeneration; Auctus Biologics: company offering orally bioavailable antibody mimetic platform; Lento Bio: company seeking AGE breakers for presbyopia and metabolic disease
Matthew Scholz, Oisin Bio
Purpose: Oisin targets age-related loss of muscle/frailty, accumulation of unwanted fat, and accumulation of senescent cells
How? Proteo-lipid vesicles: vesicles containing small viral fusion proteins, coated by (well-tolerated) lipids.
They build follistatin gene therapies for muscle growth; selectively kill adipocytes (fat cells) with genetic targeting; increase mitochondrial transcription factor A (TFAM) abundace to enhance mitochondrial activity.
Reason, Repair Bio
Purpose: Reverse atherosclerosis
How? Degrade localized excess amounts of the toxic free cholesterol, with the help of LNP-mRNA therapies
Right from the beginning, the title of Reason’s presentation (“On the Prospects for Conducting a First-in-Human Clinical Trial to Reverse Atherosclerosis in Próspera”) caught our attention. The first speaker to bring up the opportunities Próspera enables! Why? A Próspera approach for pretty much the same trial structure would be much cheaper and advantageous
Andy Lee, Vincere Biosciences
Purpose: Break down dysfunctional mitochondria (i.e., induce mitophagy) with molecules
How? USP30 inhibitor molecules
USP30 is a mitochondrial deubiquitinase; damaged mitochondria thus do not undergo ubiquitination-mediated mitophagy. Administering inhibitors of this protein will, intuitively, induce mitophagy.
Not only does USP30i induces mitophagy in the brain, but it also has therapeutic potential for kidney injury and disease.
3 more mentions:
Nils Regge from Apollo Health Ventures, Robin Mansukhani from Deciduous Therapeutics, and Sebastian Brunemeier from ImmuneAge Bio. All 3 worth following up on!
Despite only having a few moments on stage, these speakers knew how to captivate the audience. Each word carefully chosen, each slide masterfully designed. But the one thing they all shared/had in common?
The Vitalian mentality: embrace the impossible/problem, and build until the impossible is done. This is what we need to see more of, in longevity: good scientists who become good storytellers about their work.