Nothing stays the exact same on the temporospatial scale of a cell. There is no such thing as empty space, let alone space where nothing is happening. Molecules vibrate and change conformations, collide and interact with one another, consuming and dissipating energy. Is it even a surprise? A cell holds 42 million protein molecules, connected in networks (pathways) heavily responsive to the intracellular and extracellular environment. Constant movement is more than a beautiful show; it is a necessity for any process to go forward.
Does everything need to be controlled in the cell, from transport inside/outside of the cell to cytoplasmic and intra-organelle processes? While we do not have a definitive qualitative yes/no answer, we have been wrong about certain things being (not) controlled before. One of them is cell growth. Previously thought to be a spontaneous event triggered by nutrient availability, one molecule discovered on Easter Island proved us wrong.
Here comes this molecule… Rapamycin.
Rapamycin was naturally discovered in bacteria from the soil of Easter Island and was later approved by the Food and Drug Administration (FDA) as an immunosuppressant drug. To date, it is renowned for its longevity-promoting effects, backed up by strong experimental support. Molecularly, rapamycin inhibits cell growth, cell cycle progression and cell proliferation. How does rapamycin work? Let’s take a look at a cellular protein called mTOR.
1. What is mTOR?
Even though its name is bound to its inhibitor (mammalian / mechanistic Target of Rapamycin), mTOR is a central proteins discovered in the 90s. When nutrient (“food is present”) signals and mitogen (“division is possible”) signals are received, mTOR upregulates protein and lipid synthesis, downregulates autophagy (removal of unnecessary or dysfunctional cell components), thus promoting growth and proliferation. On the contrary, when energy levels drop, mTOR signals to the cell to preserve itself, to focus on the most relevant processes for survival, rather than to produce new components. Broadly, looking at the mTOR signaling pathway, countless processes are indirectly controlled: translation initiation and lysosomal biogenesis, rRNA and tRNA production as well as cytoskeletal rearrangement and general metabolism.
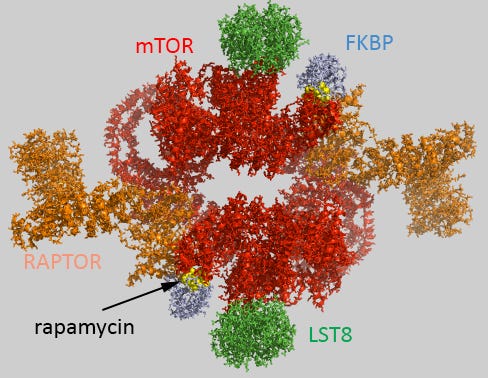
mTOR is a protein kinase found in two distinct complexes inside the cell, intuitively named mTOR Complex 1 (mTORC1) and Complex 2 (mTORC2). The two complexes are 70% structurally similar, with a primary difference in the binding of either Raptor (forming mTORC1) or Rictor (forming mTORC2) protein sub-components.
One of the first studies to investigate the difference between the two complexes (published in Cell exactly 30 years ago, back in 1993) was via gene knock-outs in yeast cells. A knock-out of the TOR1 gene led to no observable changes in the cells, suggesting that the TOR2 gene was functionally sufficient to make-up for the loss of TOR1. A knock-out of TOR2 on the other hand led to the death of the yeast cells, but not in the G1 phase (the cell cycle phase when cell growth takes place). This tells us that the cells were doing all fine… in terms of growth. Now, a knock-out of both TOR1 and TOR2 led to cell death in the G1 phase, implying that cell growth necessitated these proteins. The final conclusions inferred in this study are: TOR1 is necessary for G1 progression. This function overlaps with that of TOR2. However, TOR2 has essential non-G1 functions beyond this one. Soon, it was proven that two separate pathways exist. One is the TOR2-unique pathway (such a self-explanatory name), signaling e.g. spatial growth through cytoskeletal proteins. The second pathway is common to the two TOR complexes, signaling growth in cell mass when nutrients are present.
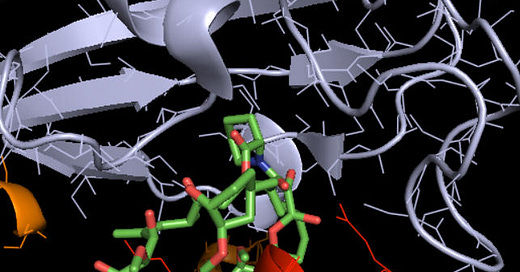
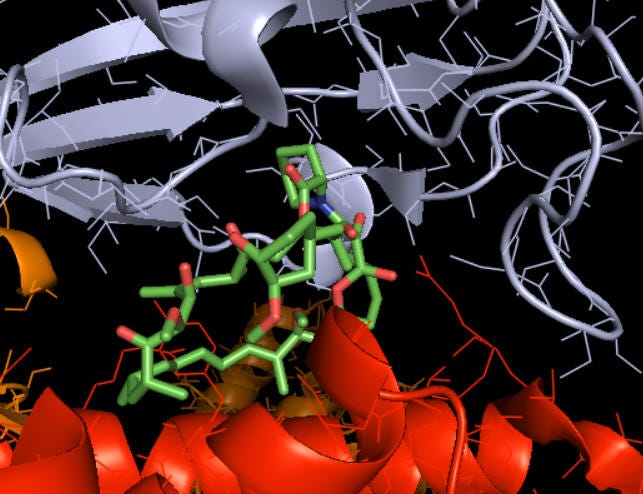
Figure 1. a) Rapamycin, shown in sticks, bound to the two mTORC1 components it interacts with (mTOR protein in red and FK Binding Protein 12 in grey). b) Zoom out of the same interaction, showing all mTORC1 protein components. The molecular reason why rapamycin cannot bind mTORC2 is that the latter lacks the key component FK Binding Protein 12. Credits: Henry Jakubowsk, “Biochemistry online”.
Interestingly, in normal conditions, rapamycin can bind mTORC1 but not mTORC2. There is one exception: under chronic (long-term) administration of rapamycin in some cell types, rapamycin can bind Complex 2 as well. Why? Not something we know yet with certainty. One clue, indicated by the lab of Brain Kennedy while he was still working at the Buck Institute of Aging in 2015, is the expression of the FK Binding Protein. (Higher expression thereof leads to mTORC2 sensitivity to rapamycin, and vice versa, low expression thereof will keep both complexes insensitive.)
A common principle in aging is that growth and proliferation lead to higher mistake production possibilities, whose accumulation leads to aging – therefore, (slightly!) hindering growth and/or proliferation at a cellular level should come with its own longevity-promoting effects. After introducing mTOR as a potent regulator of these processes, it would make sense to look for alternative ways of inhibiting its activity (other than the administration of the drug).
To this end, common processes associated with mTOR downregulation are fasting and caloric restriction. Both of these dietary practices are intensively studied due to their ability to suppress mTOR-mediated pathways and decrease the speed of aging. Molecularly, fasting increases the AMP-to-ATP ratio and activates AMPK, which downregulates mTOR.
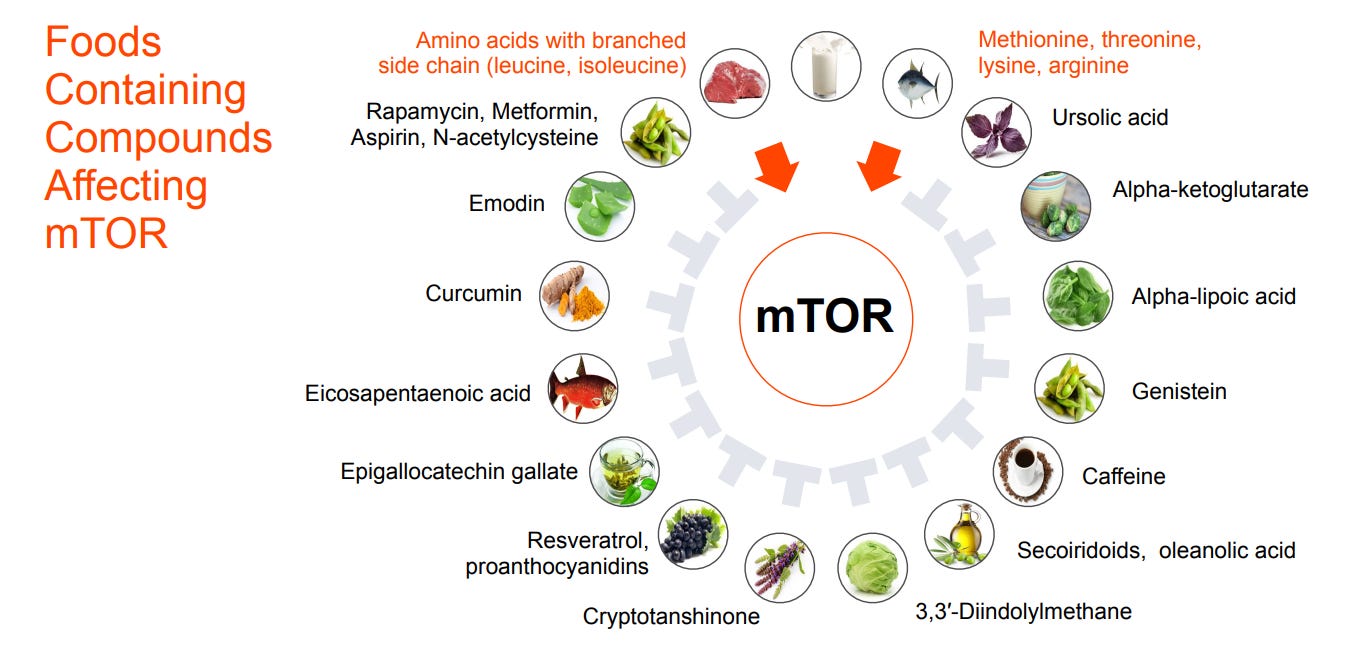
While these longevity interventions require intentionality and consistency, the good news is that unknowingly, we might consume more (or less) quantities of compounds affecting mTOR. My favorite example is caffeine as an mTOR inhibitor. (Watch me use this as an argument to justify my addictions.)
Figure 2. Common foods and the mTOR-affecting compounds they contain. Two of them are activating, while the other 14 are inhibitory. Credit: Longevity Medicine 201 course, developed by Deep Longevity.
2. What about life extension?
Rapamycin is the one of the most effective life extending molecules we now know of, with a 10-30% increase in lifespan not only in many animal models (yeast, worms, flies, mice) but dogs and monkeys too. Lifespan extension can be achieved regardless of the life stage at which the rapamycin treatment was started (early or late in life) and of the mode of administration thereof (continuous, transient, or intermitent). Rapamycin is effective over a wide dose range and, unlike many anti-aging interventions, it has life-extending effects in not just males (as it typically happens), but also females.
Anti-aging is however not about prioritizing lifespan extension, rather, healthspan extension.
Think about how mTOR fuels cell growth, and how, if not regulated, it can do so uncontrollably. Cancer is the prime example of a disease where the ability of the cells to spread and grow uncontrollably is crucial, invading surrounding tissues and compromising organ function. Thus, it may not come as a surprise that rapamycin is approved to treat renal cancer and is in trials for multiple other cancer types.
Take Alzheimer’s disease. mTOR activation facilitates among others the build-up of protein aggregates – and Alzheimer’s is known to be characterized by abnormal protein aggregates, primarily beta-amyloid plaques and tau tangles, within the brain. On a broader scale, mTOR affects long-term synaptic plasticity and long-term learning and memory consolidation. Rapamycin again could target these dysfunctions associated with Alzheimer’s disease.
Other diseases linked to disregulated mTOR include obesity, diabetes, autism spectrum disorders, lupus, and lymphoproliferative diseases.
That being said, as with any other drug, side effects have to be taken into account. For example, recall that rapamycin has immunosuppressive properties. It inhibits B- and T-cell activation by making them less responsive to an activating molecule, leading to weakened immune system. Since we are not cells living in Petri dishes nor mice living in labs, the drug administration might be even rougher on our bodies and lifestyles.
Can rapamycin be our “cure to aging”? Well, it might not be as simple as a one drugs cures all. Aging is a heterogenous anddiverse process. Its multifactorial causes build a network of targets to be hit. However, as of now, there are only promises that some fraction of these targets can be tackled. Hopefully, clinical trials will give us indications of whether the lifespan extension effects apply to us, humans, to a promising extent without many side effects. Typical questions that would then have to be tackled are, which dose of rapamycin is the most effective in achieving these positive effects? At what age could we start taking this drug? And many others.
Clinical trials are developed by Mayo Clinic, in patients (60+ y.o.) with coronary artery disease to assess safety and effects of 3 weekly doses of rapamycin. What do they look at? Frailty, quality of life (QOL), senescent-associated secretory phenotype, and adipocyte mitochondrial DNA copy number. The telemedicine platform AgelessRx has the same goals, administering a rapamycin low dose / high dose / placebo in elderly aged 50 to 85 y.o. They take into account changes in visceral fat, bone density, complete blood count, and many other biological measures.
Globally, the trials are not limited to humans: larger-breed dogs are being included too, via the NIH-funded Dog Aging Project. More details on the Test of Rapamycin in Aging Dogs (TRIAD) (part of the broader Dog Aging Project) can be found here.
In this project, some of the dogs are being administered TriviumVet’s patented veterinary Rapamycin (formally known as Felycin). In felines, another one of TritiumVet’s proprietary Rapamycin formulations is tested for ailment of Chronic Kidney Disease Ohio State University is assessing our in CKD in cats.
Overall, rapamycin has built around itself a beautiful story and record as a longevity-promoting drug. Is it going to maintain its reputation or be surpassed by rapalogs (rapamycin analogs) with better function and less side effects? For as long as clinical trials are ongoing, it remains to be seen.
Author: Denisa Lepădatu
Further references:
Michael Hall (University of Basel): The Story of TOR (Target of Rapamycin) (2017). iBiology. 6 September. Available at:
(Accessed: 10 August 2023).
Powers, T. (2022) ‘The origin story of rapamycin: Systemic bias in biomedical research and Cold War Politics’, Molecular Biology of the Cell, 33(13). doi:10.1091/mbc.e22-08-0377.
Selvarani, R., Mohammed, S. and Richardson, A. (2020) ‘Effect of rapamycin on aging and age-related diseases—past and future’, GeroScience, 43(3), pp. 1135–1158. doi:10.1007/s11357-020-00274-1.
Recommendation:
https://peterattiamd.com/davidsabatini-mattkaeberlein/?utm_source=linkedin&utm_medium=social&utm_campaign=230925-pod-davidsabatinimattkaeberlein&utm_content=-pod-davidsabatinimattkaeberlein-li
Have thoughts on this topic? We at LongX strive for collaboration on a Global Scale 🌎.
Reach out to the team at LongX and collaborate with us!